The GreenSky-backed University of Toronto spinout has set its initial sights on dentistry.
Toronto-based nanotechnology startup Mesosil has closed $2.2 million CAD in funding to scale up its production and fuel its commercialization plans.
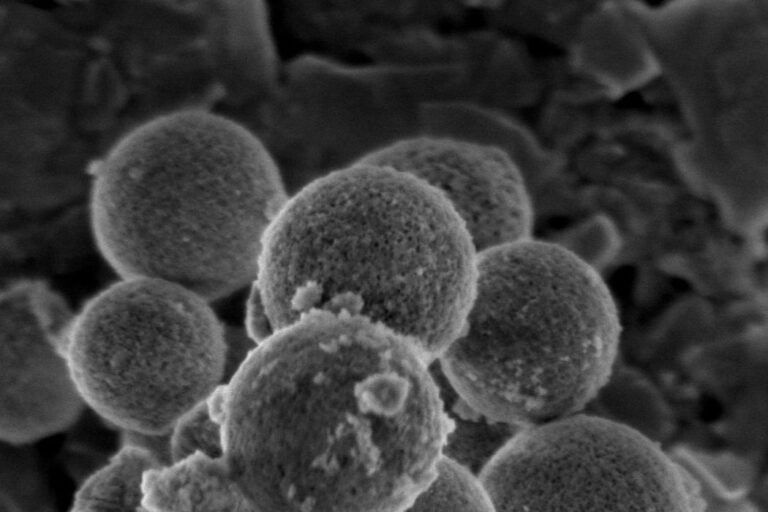
From the University of Toronto, Mesosil develops nanoscale, silicon-based additives designed to prevent infection and disease at the tissue-biomaterial level and extend the life of dental and medical devices such as fillings and implants.
After years of research and development, Mesosil has perfected its flagship material, and the startup says it’s ready to scale up production to meet customer demand in the dental space with new funding from deep-tech GreenSky Capital and a team other investors.
GreenSky CEO Marian Hoffmann described Mesosil’s progress to date as “remarkably fast and capital-efficient” for a deep-tech advanced materials company.
“We have a core product that we’re very happy with and want to acquire [it] out the door as soon as possible,” said Mesosil founder and CEO Cameron Stewart in an exclusive interview with BetaKit.
Mesosil’s equity round closed in May and was led by GreenSky with backing from new investors Greyhill Ventures, the Center for Aging + Brain Health Innovation and existing backers Archangel Network, NorthSpring Capital Partners and undisclosed angels. This capital brings Mesosil’s total funding to nearly $3.1 million.
Mesosil has developed a material that aims to reduce infections resulting from dental fillings and other implants. “Anytime you have a foreign material in the human body, whether it’s a dental filling or a hip replacement or any kind of implant, you create this boundary between material and tissue that ends up being the perfect little place for bacteria to enter and colonize. and start causing infection,” Stewart explained.
In dentistry, this can lead to a root infection or cavity adjacent to an existing filling that requires additional dental care. In orthopedics, it can lead to bone infection that requires surgery to treat, Stewart noted.
Materials like dental fillings are supposed to last a patient’s lifetime, but they are constantly exposed to bacteria and often fail within the first five years after placement, Stewart said. “If we can add something like a silicon particle that’s antimicrobial [fillings]and to give them the ability to fight back and maintain themselves, that’s a huge potential advantage,” he said.
Enter Mesosil, which has developed a tiny, “self-assembling hard sponge” filled with an antimicrobial drug designed to be released very slowly over a long period of time to kill bacteria, prevent disease and ensure that dental fillings and other implants last longer.
Born in 2015 out of Stewart’s master’s and doctoral work in a grant-funded research program at the University of Toronto School of Dentistry, Mesosil filed its first patent in 2017 and launched as an independent business in 2018. The startup began attracting interest from industry players in 2019, but has been slowed by the emergence of COVID-19 in 2020, Stewart said. Things picked up for Mesosil in 2021, and the company began a pre-seed round.
GreenSky first encountered Mesosil then and was impressed, but ultimately passed because Mesosil was early in the commercialization process, GreenSky CEO Marian Hoffmann told BetaKit. Since that meeting, he said Mesosil has achieved some “significant milestones”.
“Mesosil secured ownership of its intellectual property (IP) from the University of Toronto, has developed new IP and has worked to scale up its nanomaterials production,” said Hoffmann. “Mesosil paved its way to market, created a pipeline of commercial customers in the dental space and secured a partnership with a global dental [original equipment manufacturer].”
This progress prompted GreenSky to invest. “They have overcome the technical risk of their solution and demonstrated product-to-market fit over five years,” said Hoffmann. “As a high-tech, advanced materials company, this is an extremely fast and efficient capital process to get to market, and we’re excited to be alongside [Stewart] for the next phase of its development”.
RELATED: GreenSky expands team and secures first close of $17M Fund V as firm sees opportunity amid recession
Stewart said Mesosil wanted a local investor to lead its manufacturing cycle and was attracted by GreenSky’s track record in the chemical additives space, where it has worked with biotech and medtech companies. “There’s a confluence of experiences there that I think was a good fit,” he said. The CEO declined to disclose Mesosil’s valuation, but claimed the startup’s latest funding was bullish on its pre-launch funding in 2021.
Mesosil is currently working with companies in the professional dental space in the United States and Europe to develop solutions for infection-fighting dental materials.
Hoffmann claimed that Mesosil’s product has “the longest release duration on the market” and is unique to competitors in providing both biodegradation prevention and antimicrobial properties. Another advantage, he noted, is its compatibility with existing dental sealants.
“We’re following a lot of similar technologies that maybe started life … in dentistry and graduated to orthopedics after they proved themselves.”
Cameron Stewart, Mesosil
In addition to dental fillings and implants, Hoffman said the Mesosil solution could be applied to bone cement and antifouling coatings. Longer term, Stewart also sees potential orthopedic, other medical, and even some industrial applications for Mesosil’s technology.
Stewart noted that the initial target market for Mesosil is dentistry because the use case is clear and the risk is lower than in orthopedics, adding that problems in a patient’s mouth are easier to treat than problems in the knee .
The dental space also has a lower barrier to entry from a regulatory standpoint, he said. “We’re following a lot of similar technologies that maybe started life in the dental implant space or the dental space and graduated to orthopedics once they’re proven.”
Mesosil’s customers must obtain regulatory approval to bring their medical devices to market. The Toronto startup supports this process as it relates to its own hardware.
“What we really have to hold ourselves up to is just in terms of the quality of material that we provide,” Stewart said. “We have to make sure that every gram of material that goes out the door is at that level of medical device quality.”
Featured image courtesy of Mesosil.