Fluoride treatments aren’t just for protecting your teeth. According to a new study, an ingredient found in toothpaste may also help prevent solar cell damage (Nat. Energy 2019, DOI: 10.1038/s41560-019-0382-6). The finding may lead to simple chemical processes to extend the lifetime of low-cost photovoltaic (PV) devices based on a promising class of materials called perovskites.
Solar cells made with metal-organic perovskite materials that absorb sunlight have taken the photovoltaic world by storm in recent years. The reason is that methylammonium lead trihalides and other perovskites are much less expensive to manufacture and process than crystalline silicon, the standard PV material, but offer comparable performance. The power conversion efficiency – a measure of sunlight in and electric current – of the best perovskite cells matches that of crystalline silicon cells, about 23%. But perovskite cells do not stand the test of time. within a few days, the photoactive layer decomposes. This weakness has hindered the commercialization of this promising class of solar cells.
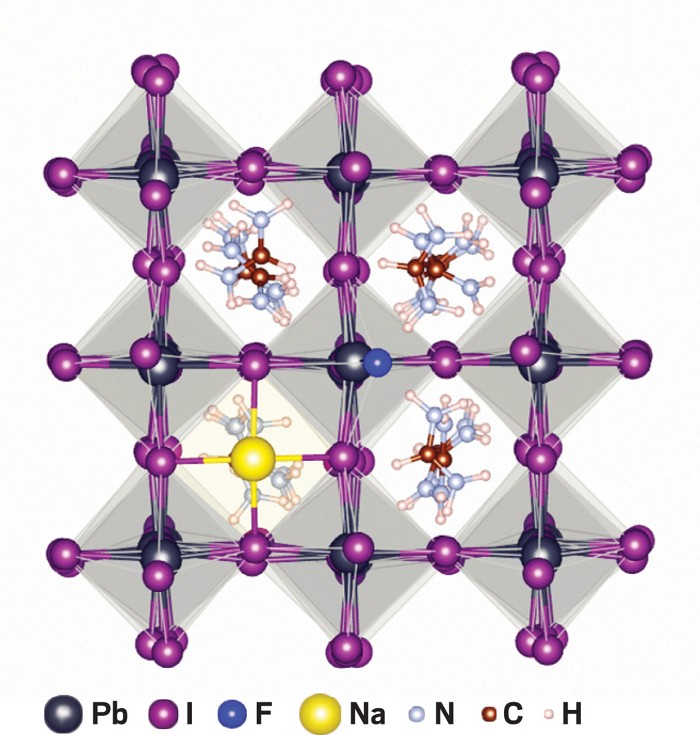
Ion vacancies in the thin crystalline perovskite layer are common initiation sites for decomposition. The presence of these defects causes the halide anions and organic cations in the material to diffuse to the surface of the film, from where they can desorb, evaporate, and undergo other reactions that degrade the perovskite layer. Moisture, heat and bright light make these reactions worse.
To eliminate ion vacancies and combat this breakdown process, Nengxu Li and Huanping Zhou of Peking University, Shuxia Tao of Eindhoven University of Technology and their colleagues treated their perovskites with various alkali halides. They compared dozens of treated and untreated cells, operating them at high temperatures and under bright light to speed up the aging process. Treatment with sodium fluoride, a common toothpaste ingredient, worked best. The fluorine-treated cells that had an initial power conversion efficiency close to 21.5% maintained their efficiency for more than 1,000 hours, while the efficiency of the untreated cells fell to half of their original value.
Based on spectroscopy, microscopy and calculations, the researchers attribute the durability of the treated cells to the high electronegativity of fluorine, which gives the ion the ability to form uniquely strong hydrogen and ionic bonds. They explain that the fluoride ions form strong hydrogen bonds with the material’s organic cations and strong ionic bonds with the lead halide, fixing these species in place and preventing decomposition.
The team’s simple fluorine treatment passivated the anion and cation vacancies in the perovskites simultaneously, which is impressive, says Yuanyuan (Alvin) Zhou of Brown University, an expert on perovskite solar cells. Previous studies have only looked at one type of defect, he says. In addition, he says the team has proposed “a very plausible mechanism” for why this works so well. It would be very interesting to use high-resolution microscopy to see exactly what happens in these fluorine-passivated perovskites, he adds.