Image
Español
People seek treatments to smooth smile lines and crow’s feet and plump up their lips, cheeks and hands.
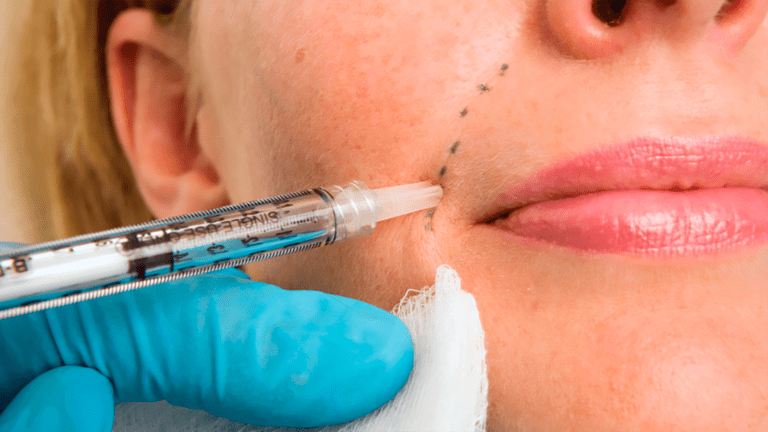
Injecting dermal fillers into the face and arms can improve the appearance of facial lines and volume loss caused by age or certain medical conditions. In studies of dermal fillers approved by the US Food and Drug Administration, people generally report being satisfied with the results of their treatment.
However, dermal fillers are not for everyone. Dermal fillers may not be suitable for people with certain medical conditions, such as bleeding disorders or certain allergies. If your healthcare provider confirms that dermal fillers are an option for you, be aware that all medical products have benefits and risks. The FDA advises you to work with a licensed health care provider who is experienced in injecting dermal fillers, knowledgeable about fillers, anatomy, managing complications, and most importantly, informs you of the risks and benefits before you receive treatment.
What are dermal fillers?
Dermal fillers are gel-like substances that are injected under the skin. Dermal fillers are intended to create a smoother or fuller appearance, or both.
The FDA regulates dermal fillers as medical devices. As reported in clinical trials, the results of most FDA-approved dermal fillers are temporary because they are made of materials that the body eventually breaks down and absorbs. The injection process may need to be repeated to maintain the desired effect.
Types of dermal fillers
Temporary filling materials include the following materials:
- Hyaluronic acid, a sugar found naturally in the body
- Calcium hydroxylapatite, mineral and main component of bone
- Poly-L-lactic acid (PLLA), biodegradable, synthetic material
There is only one FDA-approved dermal filler that is not absorbed by the body. It is made with polymethyl methacrylate (PMMA) beads suspended in a solution containing bovine (cow) collagen. PMMA beads are tiny round, smooth, plastic beads.
FDA approved uses of dermal fillers
Dermal fillers are approved for specific uses in people 22 years of age and older. These uses include:
- Correction of moderate to severe facial wrinkles and skin folds
- Increasing the fullness of the lips, cheeks, chin, hollows under the eyes, jaw and back of the hand
- Reversal of facial fat loss in people with human immunodeficiency virus (HIV)
- Correction of acne scars on the cheek
FDA warnings about unapproved fillers
- The FDA has not approved injectable silicone or any injectable filler for body contouring or enhancement. The FDA has warned against injecting filler into the breasts, buttocks, or spaces between muscles. The use of injectable filler for large-scale body contouring or body enhancement can result in serious injury, including long-term pain, infection, permanent scarring or disfigurement, and even death.
- The FDA has not approved needle-free devices for injecting dermal fillers and warns against using them to inject hyaluronic acid or other lip and facial fillers. Injectors use high pressure and do not provide enough control over where the filler will be placed. Serious injuries and in some cases permanent damage to the skin, lips or eyes have occurred.
- The FDA also warns against buying or using lip or facial fillers sold directly to the public. They are not FDA approved and may be contaminated with chemicals and pathogens. The only FDA-approved dermal fillers are available by prescription for injection by a licensed healthcare professional using a syringe with a needle or cannula (a small, blunt-ended tube inserted under the skin).
Risks of FDA-approved fillers
As with any medical procedure, there are risks with the use of dermal fillers. Most side effects associated with dermal fillers, such as swelling and bruising, occur immediately after injection, and many resolve within days to weeks. In some cases, side effects may appear weeks, months or years later.
Common risks include:
- Bruises
- Redness
- Swelling
- Pain
- Tenderness
- Itching
- Skin rash
- Difficulty performing activities (seen only when the back of the hand is injected)
Less common risks include:
- Inflammation such as swelling or redness may develop near the injection site of the dermal filler after viral or bacterial illnesses or infections, vaccinations or dental procedures
- Raised lumps in or under the skin (nodules or granulomas) that may need to be treated with injections, oral antibiotics, or surgical removal
- Contamination
- Open or draining wounds
- Injection site wound
- Allergic reaction
- Necrosis (tissue death)
People should be tested for allergies before receiving dermal fillers made with certain materials, especially animal-derived materials such as collagen.
Seamless injection into blood vessels
The most serious risk associated with dermal fillers is accidental injection into a blood vessel. The filler entering a blood vessel can cause skin necrosis (tissue death), stroke or blindness. Although the chances of this happening are small, if it does, the resulting complications can be serious and permanent.
Removal of dermal fillers
If you want to remove or reduce fillers due to side effects, you may need additional procedures to reduce the filler or surgery to remove it. These procedures carry their own risks. Please note that some fillers may be difficult or impossible to remove.
6 Tips for Consumers About Injectable Dermal Fillers
- Work with a licensed health care provider experienced in the fields of dermatology or plastic surgery and trained in injecting dermal fillers. The provider should use properly labeled, sealed vials or prefilled syringes of FDA-approved filler.
- Request and read the patient labeling information on FDA-approved injectable dermal fillers from your authorized healthcare provider.
- You know the type of product to be injected and the potential risks. Know where each product you receive will be injected. Talk to your licensed health care provider if you have any questions.
- Do not buy dermal fillers sold directly to the public. They may be counterfeit, contaminated, or not approved for use in US FDA-approved dermal fillers that are only for prescription use.
- Do not inject yourself with dermal fillers or “pen” for injection without a needle.
- Do not inject any type of filler or liquid silicone for body contouring.
Dermal Fillers and Botulinum Toxin Products
The FDA has also approved botulinum toxin products such as Botox, Dysport, Xeomin, and Jeuveau to treat facial wrinkles. These products are not dermal fillers. They are injectable drugs that work by preventing the muscles from tightening, making wrinkles less visible. The safe use of dermal fillers in combination with Botox and other treatments has not been evaluated in clinical studies.
Although botulinum toxin products are derived from the same bacteria that cause botulism, the amounts used for cosmetic purposes are purified and many orders of magnitude smaller.
The FDA has approved these injectable medications to temporarily improve the appearance of one or perhaps several types of facial lines, including frown lines, forehead lines, and crow’s feet.
Side effects reported in clinical trials include facial weakness, eyelid ptosis, and eyebrow droop. Other side effects included localized pain, swelling, redness, and bruising at the injection site. In rare cases, injections have resulted in double vision, dry eyes, or difficulty swallowing or breathing. Injecting botulinum toxin products for cosmetic purposes is not recommended for use during pregnancy or lactation.
More information
If you’ve had a problem with a dermal filler or other FDA-regulated product, you can voluntarily report it to MedWatch, the FDA’s safety and adverse event reporting program.