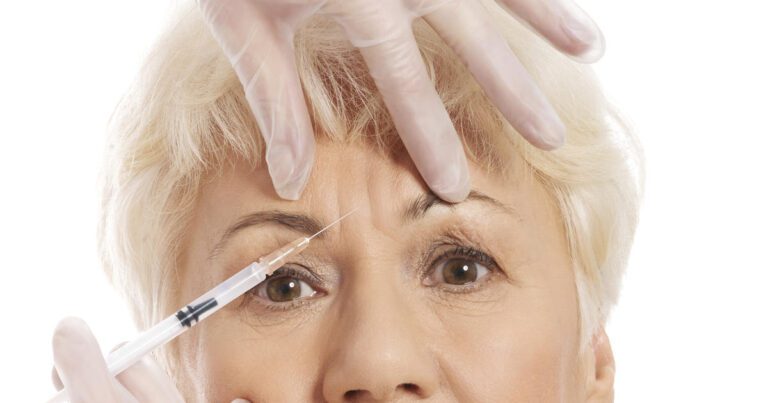
Federal officials are trying to determine the source of Botox injections — some of which may be fake — linked to an outbreak of botulism-like illnesses in several U.S. states.
The Food and Drug Administration told CBS MoneyWatch that it is working with other federal and state agencies to determine the cause of an outbreak that has sickened at least six people in Illinois and Tennessee who were injected with the botulism toxin.
Tennessee Department of Health was mentioned Four people in the state had been struck down with botulism-like symptoms, including two hospitalized after suspected bogus injections.
In Illinois, health officials are located warning medical providers to be alert for botulism patients after two people became ill and were hospitalized with symptoms including blurred vision, droopy face and difficulty breathing. Both received injections from a LaSalle County registered nurse who administered treatment without a license.
The Centers for Disease Control and Prevention said the botulinum toxin injections — commonly called Botox — were given in “non-medical” settings and “the sources of these botulinum toxin products are unknown or unverified.”
AbbVie and Allergan Aesthetics are the only authorized suppliers of Botox, and recent reports of possible cases of botulism involved “suspected counterfeit” product, Allergen, the FDA-approved maker of Botox, told CBS MoneyWatch. “Working with public health authorities, we have confirmed the safety of the Botox and Botox cosmetic supply chain, as well as the safety, quality and efficacy of all the products we manufacture and distribute,” Allergan said.
Approved for cosmetic use more than 20 years ago, Botox is popular drug used to smooth wrinkles and look younger, with injections typically costing about $530, according to the American Society of Plastic Surgeons. The results of one shot last three to four months on average, so additional shots are needed to remain wrinkle-free.
Botulism is serious and sometimes fatal disease caused by a toxin that can be transmitted through food or result from untreated wounds, while infants can develop an enteric form of the disease, according to the CDC.
So-called iatrogenic botulism is caused by overexposure to botulism toxin, although confirmed cases that occur after cosmetic or therapeutic injections are rare, according to health officials. Injections should include an FDA-approved product administered by a licensed provider, health experts advise.
The FDA urged people who experience side effects or health care providers who treat patients with side effects to report them in the FDA MedWatch reporting program.
Federal officials have previously cracked down on unregulated Botox and other cosmetic treatments. In 2023, US Customs and Border Protection officials in Ohio intercept such fillers which had been sent from Bulgaria, China, Korea and Spain.